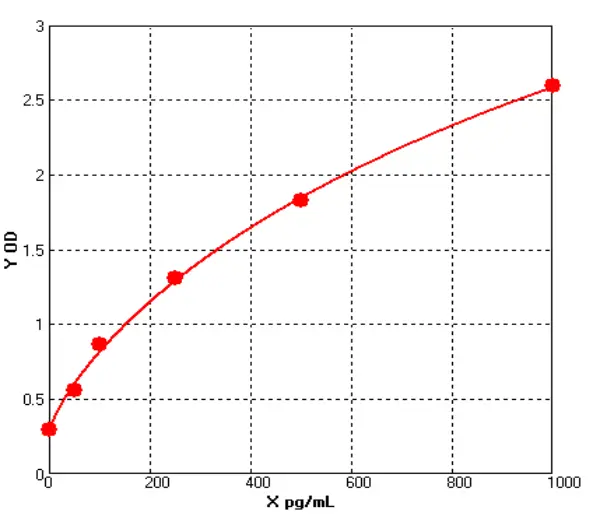
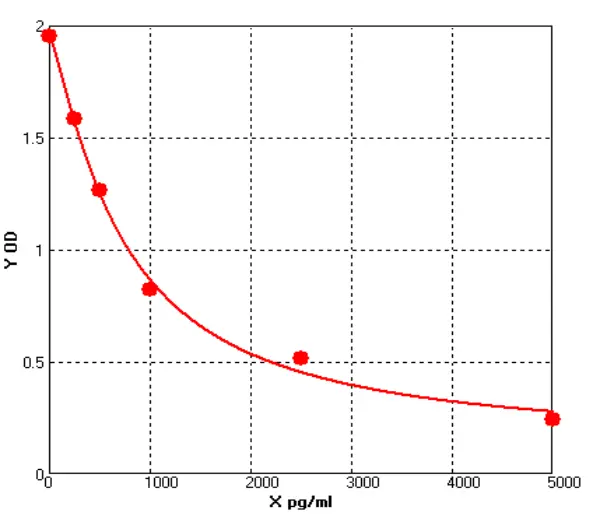
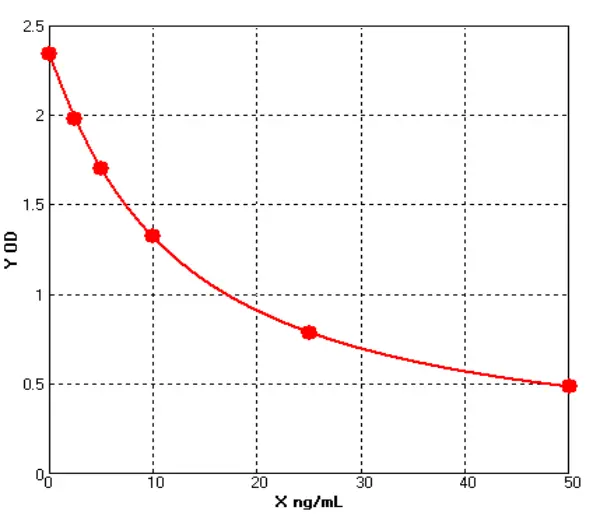
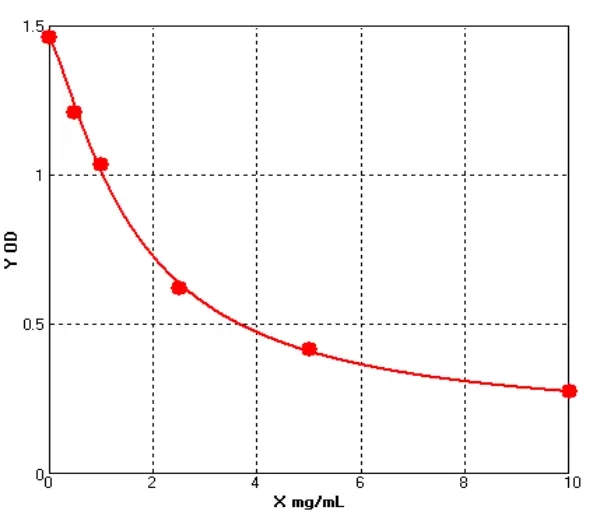
E01S0009 Human Carcinoembryonic Antigen ELISA kit
The Human Carcinoembryonic Antigen ELISA kit can be used to identify samples from the human species. Carcinoembryonic Antigen can also be called CD66E, CD66, CEACAM5, Carcinoembryonic Antigen-related Cell Adhesion Molecule 5, Carcinoembryonic Antigen.