Search ELISA Kits
Bluegene Biotech/Cellgene Bioscience Host Cell DNA Residue Detection Kits Types
What is residual host cell DNA?
-
Host cell residual DNA refers to the small amount of nucleic acid (host genome, plasmid, or total DNA) that remains in protein drugs expressed and purified through cell culture. During the production process of expressing antibody protein drugs, a large number of cells undergo proliferation and amplification, and some aging cells die and lyse, releasing a large amount of genomic DNA. This leads to the presence of a large amount of free host cell DNA in the cell culture harvest of recombinant biologics, and a small part of the host cell DNA is removed during the processing steps (such as acid precipitation, filtration, etc.), while most of it is removed during the anion exchange chromatography step. Finally, there is a trace amount of host cell residual DNA in the original solution of the recombinant protein drug.
What are the methods for residual DNA quantification?
-
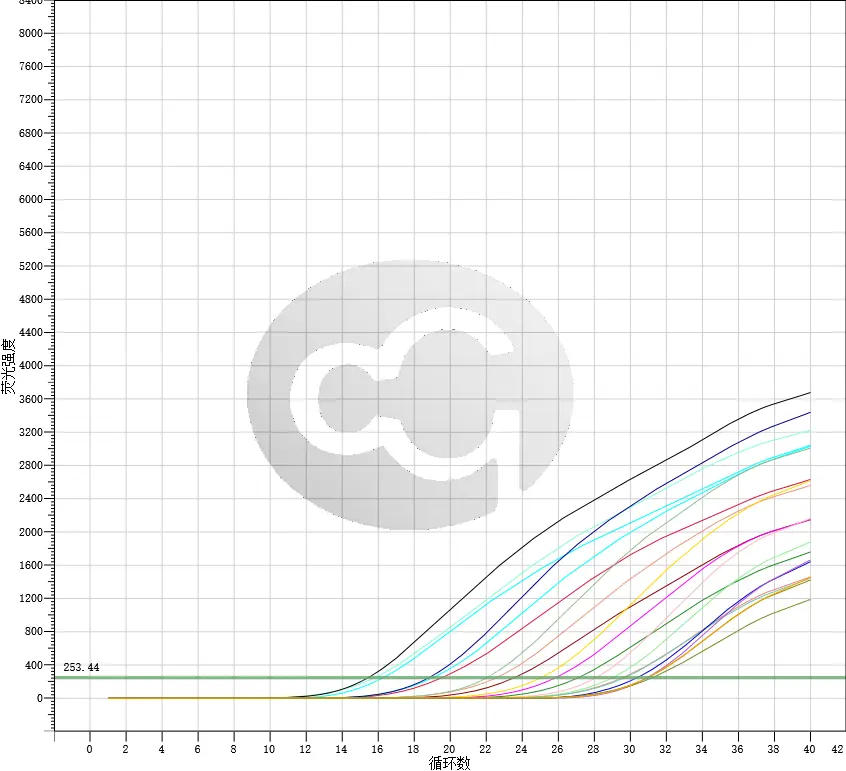
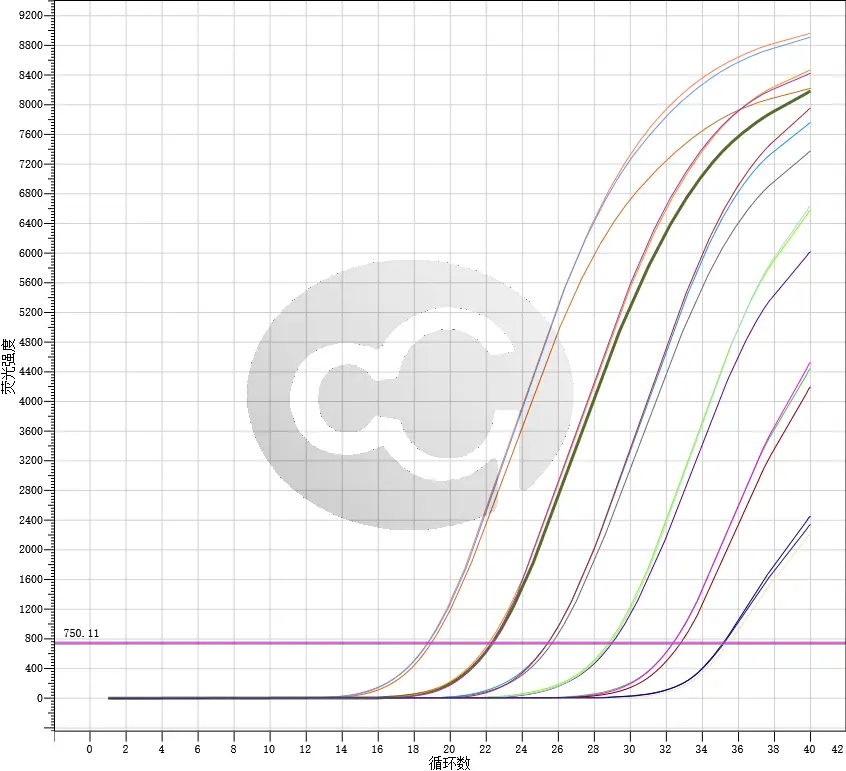
Current detection methods for host cell residual DNA in biologics include DNA probe hybridization, fluorescent dye staining, Threshold immunoassay, real-time quantitative PCR, and Whole genome amplification (WGA).
How do you remove host cell DNA?
-
Different purification steps in the production process normally all have good removal effects on host residual DNA, among which the removal effect of anion exchange chromatography is the most significant. This provides strong support for the development, optimization, and process validation of the purification process of antibody drugs.
What is the most accurate method for DNA quantification?
-
The latest revised USP (draft) General Chapter only retains the Q-PCR method, and the Pharmacopoeia of the People's Republic of China also plans to include the Q-PCR method for detection of host cell residual DNA. This method has high specificity and sensitivity, making it a reliable and widely used method in biologics quality control.
Why use this assay kit?
-
It has been specifically designed in accordance with the strictest requirements set forth by the FDA and ICH guidelines, with qPCR being the sole recognized detection method by the FDA. This ensures accurate and reliable results for your research or diagnostic needs.
ICH HARMONISED TRIPARTITE GUIDELINE
Specifications: Test Procedures And Acceptance Criteria For Biotechnological/Biological Products Q6B