Search ELISA Kits
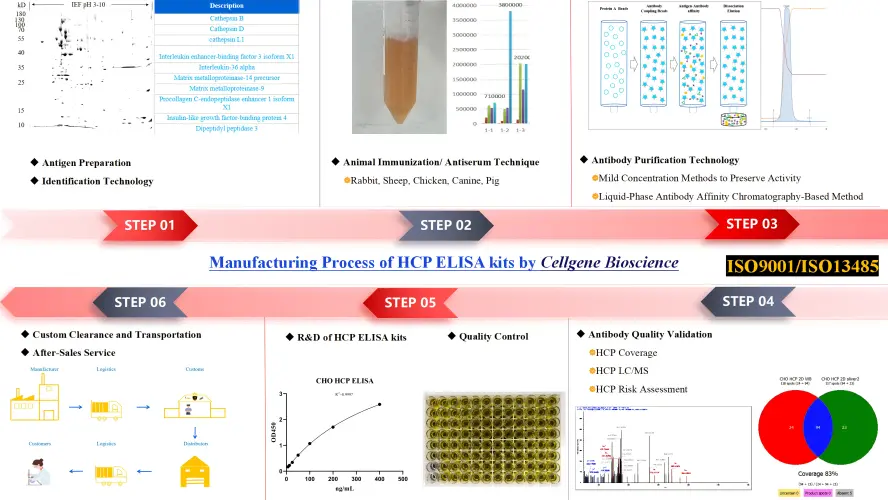
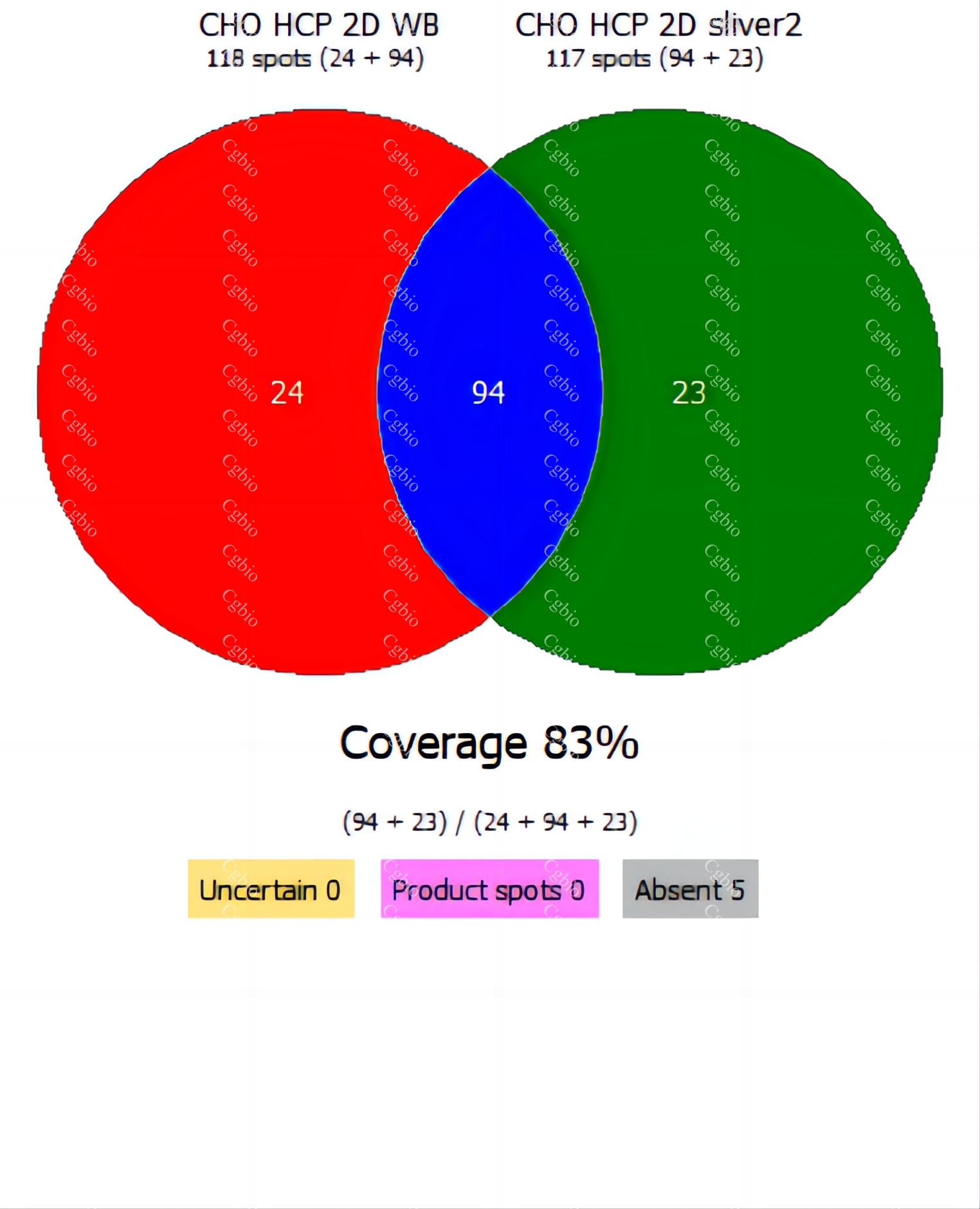
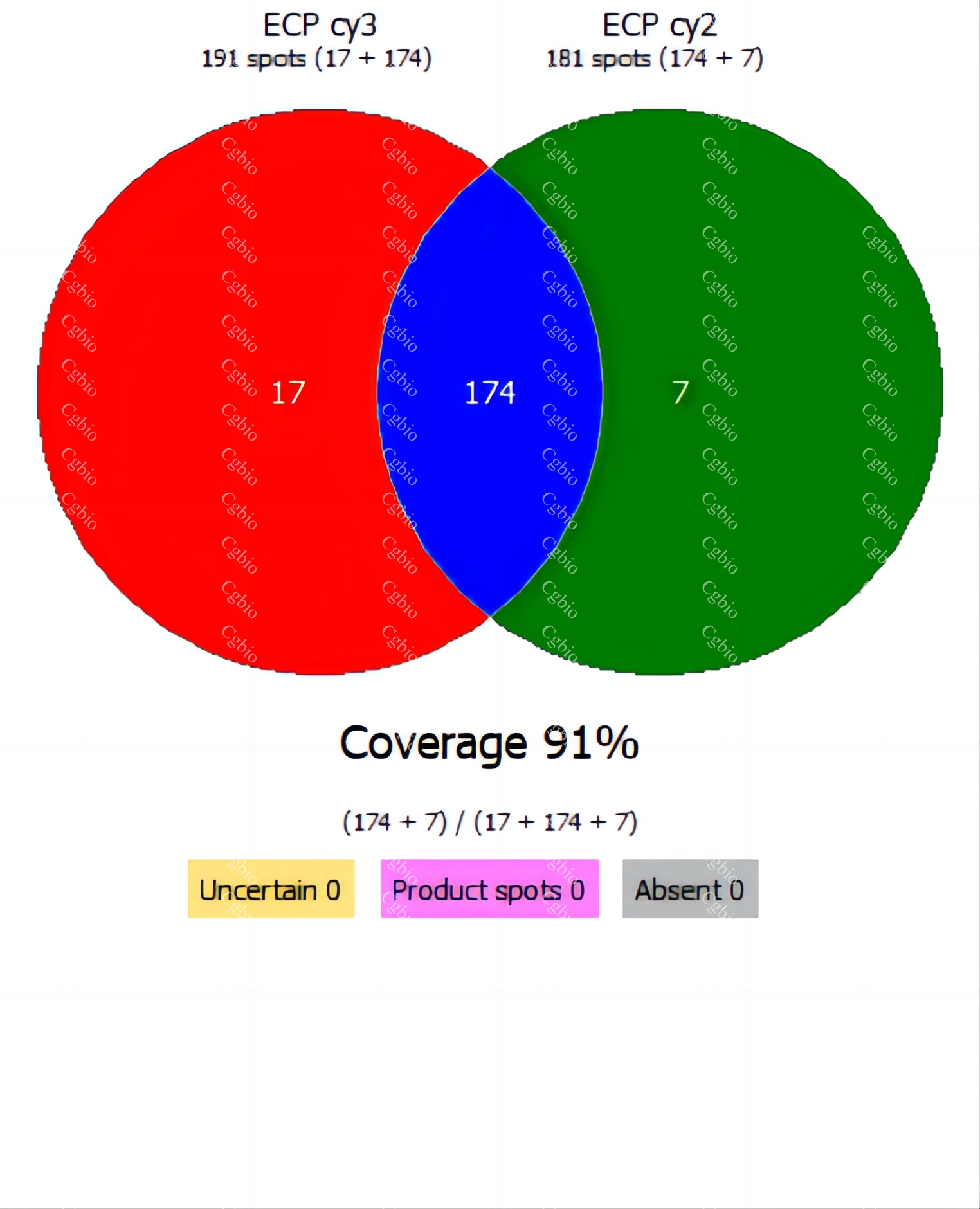
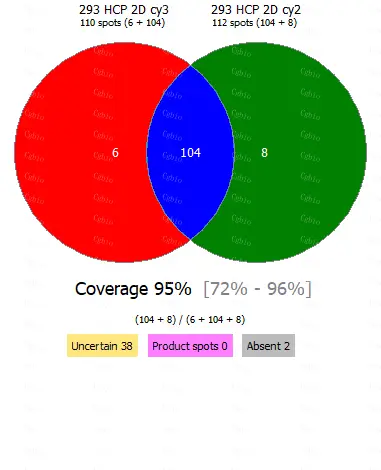
Bluegene Biotech/Cellgene Bioscience Host Cell Protein ELISA Kits Types
Requirement of the Regulation (FDA, EMA, ICH)
-
FDA, EMA, and ICH all have precise regulations for the Host Cell Protein residues.
FDA requires that potential contaminants (including Host Cell Proteins ) introduced by the purification process should be below detectable levels using a highly sensitive method.

EMA also made a statement, Residual host cell DNA and residual host cell proteins are among the impurities to be eliminated, for HCP, whatever the product and production system, residual HCP has to be tested for on a routine basis.

ICH: For host cell proteins, a sensitive assay is utilized, and the removal of cell substrate-derived impurities such as HCP may sometimes be used to eliminate the need for establishing acceptance criteria for these impurities.

Examples of HCP Related Issues with Biologics
-
In 2006, an immunogenicity issue occurred to Omnitrope (rHGH biosimilar), the cause of which was linked to excess host cell protein contamination.
In 2008, Alpheon and Roferon-A finished products were also investigated for the composition of the different impurities that were not fully studied.
In 2013, IB1001 was a recombinant product for treatment and prevention, the clinical studies of which were on clinical hold due to a higher-than-expected rate of host cell antibody development in people treated with IB001.