Comprehensive Analysis of Host Cell Protein (HCP), G3 Detection Kit in Chinese Hamster Ovary (CHO) Cells
1. Research Background
In the current biopharmaceutical pipeline, the main drug forms for the treatment of inflammation, cancer, and infectious diseases are monoclonal antibodies. The main antibody drug targets are TNF-α, VEGF/VEGFR, PD-1/PD-L1, HER2, IL12/IL23, IL17/IL17R, and so on. In 2019, the market reached $140 billion, and it is increasing year by year.
Antibody drugs developed from the initial murine antibodies, to chimeric antibodies in humans and mice, and then to humanized and fully humanized antibodies, and derived Fab antibodies, single domain and single chain antibody forms. Nowadays, innovative antibody drugs such as small molecule antibodies, antibody conjugated drugs, antibody fusion proteins, bi-specific and multispecific antibodies have also been derived from monoclonal antibodies.
Common protein drug expression systems include prokaryotic expression systems, such as Escherichia coli, E.coli; Plant expression system; Fungal expression systems, such as Pichia pastoris, Saccharomyces cerevisiae; Insect expression systems, such as Spodoptera frugiperda cells, SF9 insect cells; Mammalian cell expression systems, such as Chinese hamster ovary cells (CHO), African green monkey kidney cells (Vero), etc. Mammalian cell expression systems are widely used in the production of clinical drugs. Subsequently, we introduced the common protein expression system and its strain characteristics, the packing media used for protein purification and its application range, as well as the impurity residues that should be paid attention to in the process of protein purification, and the ingredients that are pathogenic or reduce the activity and stability of proteins (research progress of detection methods containing impurity residues).
Taking CHO as an example, HCP produced by CHO cells is diverse and complex [2]. Hamster and human have 80% common genes, CHO HCP and human endogenous protein have homology, residual HCP will also cause autoimmune reaction, and cause cross-reaction with human own protein [3,4].
2. Regulatory Requirements
After the antibody drug is expressed, downstream purification operations such as culture supernatant collection are performed. Downstream operations are designed mainly for Host cell protein (HCP), nucleic acids (plasmids, host genome, RNA), lipids, and product-related impurities [5]. The final product had HCP levels <100 PPM, residual DNA<10ng/ dose, and a high molecular weight immune total of <5% [6].
3. Introduction of Total Proteins of Host Cells
Total proteins of host cells: In the analysis of HCP, according to the role of HCP in the product, it can be divided into four categories [121]
1) Proteins with high biological activity. Impurities in it, such as the transforming growth factor β1 secreted by CHO-K1, have strong multiple effects on multiple cell types and have immune-inducing activity on human cells [7]
2) Enzymes that have a decomposition effect on product proteins or product protectors. In macromolecular formulations, they improve the stability of proteins during production, transportation, and storage [8]. For example, Tween 80 can be cleaved by phospholipases at the molecular lipid bonds, decomposing into fatty acids and alcohols, leading to the loss of drug protective activity.
3) A protein that is highly immunogenic to the host, has an adjuvant effect, and induces inflammation and immune responses. Induces the release of cytokines and leads to the up-regulation of cell surface markers associated with the antigen presentation process.
4) A type of protein that has no effect on the body or the product protein, but can be co-bound and purified with the product protein.

4: The Introduction of the Classical Method of HCP Detection
The classical methods for HCP detection include two-dimensional electrophoresis, protein spectrometry and enzyme-linked immunosorbent assay. The FDA requires that the three methods be mutually verified in order to obtain accurate test results.
Innovative methods have also been introduced, such as Liquid Chromatography-tandem Mass Spectrometry, LC-MS/MS) and the Immunoassay platform ELLA and Electrochemiluminescence immunoassay (ECLIA).
At present, ELISA assay relies on its excellent specificity, sensitivity, and high throughput, and is still the gold standard for HCP detection. At the same time, LC-MS and 2D methods were used.

Based on the ELISA method, Cellgene Bioscience has focused on the research and development of HCP products for 10 years, and can provide the following whole-process specialized technical services.
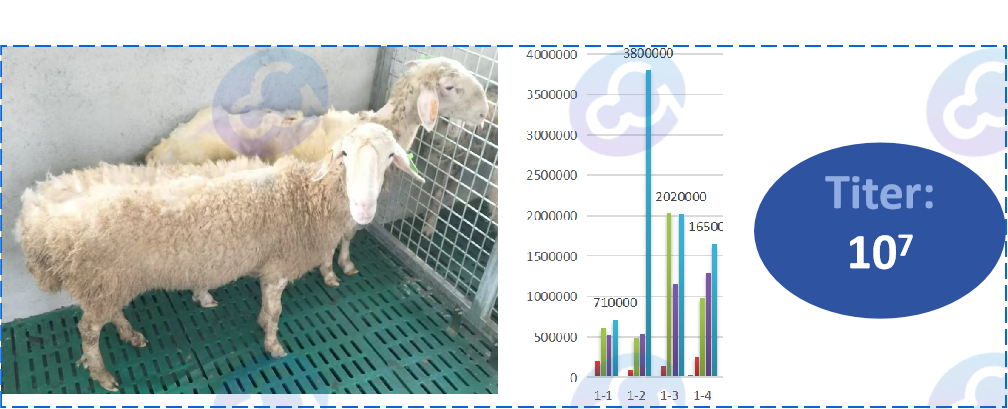
1) Immunity and Antibody Preparation
Animal immunity is protein antigen, with the aid of immune adjuvant coated antigen solution, the antigen is delivered to the skin, through T help cells to present the antigen to B cells, and form plasma cells, secreting specific antibodies into the blood. Young female rabbits were immunized subcutaneously with HCP protein antigen and adjuvant, and serum titer was detected by enzyme-linked immunosorbent assay (ELISA) two weeks later, and serum antibodies were collected through enhanced immunization or arterial blood sampling and slaughter. After immunization, antibody titer can reach 106-107 level. The amount of antiserum can reach tens to hundreds of milliliters.
2) Purification and Antibody Labeling
Arterial blood collection, 4℃ overnight, centrifuge supernatant, that is, antiserum. After salting out, Protein A/G affinity chromatography, non-igg proteins flow through, IgG binds to the Protein A domain through the Fc end, excess binding buffer, low pH elution after equilibrium, with sharp UV elution peaks. The eluent is collected and balanced to a neutral pH. After buffer displacement, concentration, Nanodrop One C UV and Bradford assay, biotin or horseradish peroxidase were labeled for HCP binding validation.
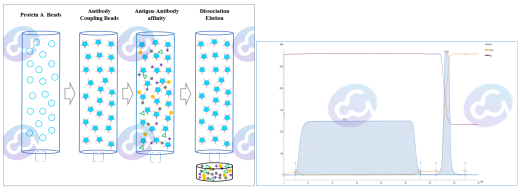
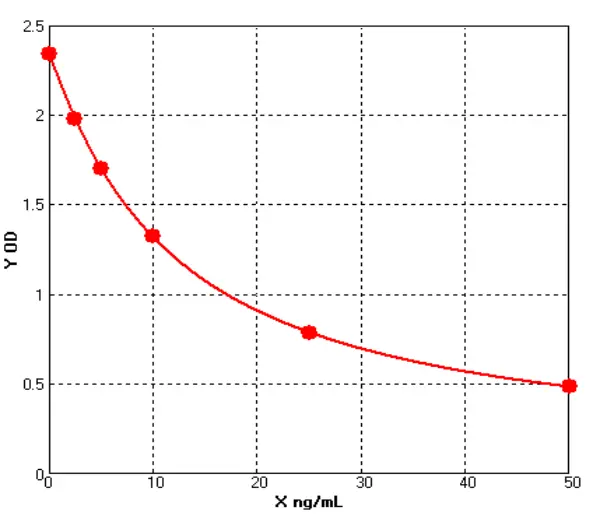
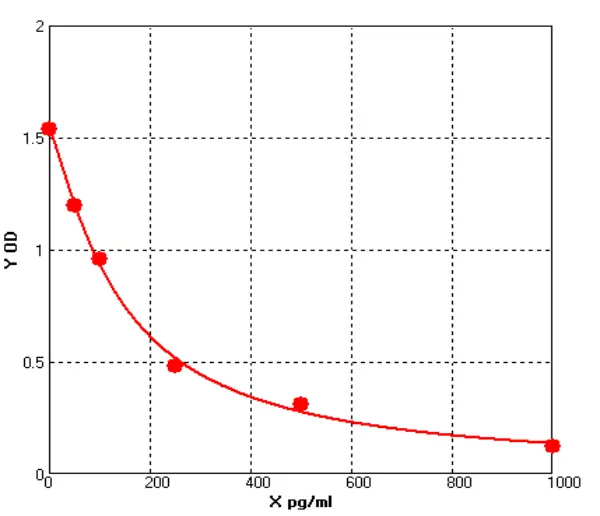
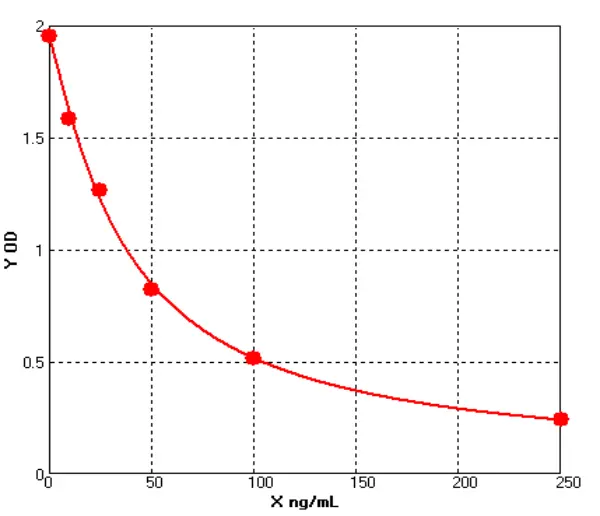
3) Kit of QC Verification
(1). Standard Curve

(2). LOD/LOQ

(3). Recovery Rate

(4). Stability
Accelerated destruction experiments showed that the kit could be stored at 4 ° C for one year. The shelf life is 6 months.

(5). Sample Detection
The drug product samples and cell supernatant samples showed that the drug product was basically superior to the imported brand, and the cell culture supernatant was superior to the imported brand. Note: Since the cell culture supernatant is provided by Cellgene Bioscience, further comparison can be made with a third party.

5: Company Introduction
Shanghai Cellgene Bioscience Co., LTD., focusing on 10 years of research and development in the field of biomedical industry testing, launched a series of HCP residue detection products, as well as HCP specific antibody preparation, coverage analysis and other whole-process technical services.
Reference:
[1] Chadd HE, Chamow SM. Therapeutic antibody expression technology[J]. Current Opinion In Biotechnology, 2001, 12(2): 188-194.
[2] Smales C M, Dinnis D M, Stansfield S M, et al. Comparative proteomic analysis of GS-NS0 murine myeloma cell lines with varying recombinant monoclonal antibody production rate[J]. Biotechnology and Bioengineering, 2004, 88(4):474-488.
[3] Gutiérrez AH, Moise L, De Groot AS. Of [hamsters] and men: A new perspective on host cell proteins[J]. Human Vaccines & Immunotherapeutics, 2012, 8(9):1172-1174
[4] Kanade Shinkai, Markus Mohrs & Richard M. Locksley. Helper T cells regulate type-2 innate immunity in vivo[J]. Nature, 2002, 420:825-829.
[5] USP:Guidance for Industry Q6B: Specifications
[6] 中国药典
[7] Beatson R, Sproviero D, Maher J, et al. Transforming growth factor-b1 is constitutively secreted by Chinese hamster ovary cells and is functional in human cells[J]. Biotechnology and Bioengineering, 2011, 108(11):2759-2764.
[8] M. Hiraoka et al. Cloning and characterization of a lysosomal phospholipase A2, 1-O-acylceramide synthase[J]. J Biol Chem, 2002, 277(12):10090-9.