E01I0389 Human Interleukin 35 ELISA kit
The Human Interleukin 35 ELISA kit can be used to identify samples from the human species. Interleukin 35 can also be called Interleukin-35, IL35, IL 35, IL-35, Interleukin 35.


E01I0389 Human Interleukin 35 ELISA kit
The Human Interleukin 35 ELISA kit can be used to identify samples from the human species. Interleukin 35 can also be called Interleukin-35, IL35, IL 35, IL-35, Interleukin 35.
Product Information | |
Cat. No. | E01I0389 |
Product Name | Human Interleukin 35 ELISA kit |
Species | Human |
Product Size | 48 Tests / 96 Tests |
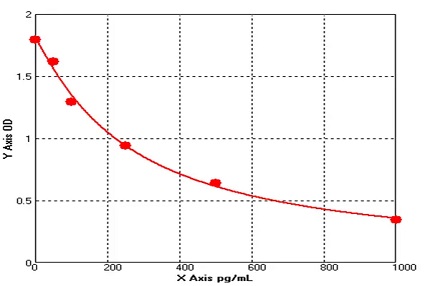
Concentration | 50-1000 pg/ml |
Sensitivity | 1.0 pg/ml |
Principal | Sandwich ELISA |
Sample Volume | 50 ul |
Sample Type | Serum, plasma, cell culture supernatants, body fluid and tissue homogenate |
Assay Time | 90 minutes |
Platform | Microplate Reader |
Conjugate | HRP |
Detection Method | Colorimetric |
Storage | 2-8°C |
Kit Components | ||
MATERIALS | SPECIFICATION | QUANTITY |
MICROTITER PLATE | 96 wells | stripwell |
ENZYME CONJUGATE | 10.0 mL | 1 vial |
STANDARD A (0.5mL) | 0 pg/mL | 1 vial |
STANDARD B (0.5mL) | 50 pg/mL | 1 vial |
STANDARD C (0.5mL) | 100 pg/mL | 1 vial |
STANDARD D (0.5mL) | 250 pg/mL | 1 vial |
STANDARD E (0.5mL) | 500 pg/mL | 1 vial |
STANDARD F (0.5mL) | 1000 pg/mL | 1 vial |
SUBSTRATE A | 6 mL | 1 vial |
SUBSTRATE B | 6 mL | 1 vial |
STOP SOLUTION | 6 mL | 1 vial |
WASH SOLUTION (100 x) | 10 mL | 1 vial |
BALANCE SOLUTION | 3 mL | 1 vial |
Principle of the Assay |
IL 35 ELISA kit applies the quantitative sandwich enzyme immunoassay technique. The microtiter plate has been pre-coated with a monoclonal antibody specific for IL 35. Standards or samples are then added to the microtiter plate wells and IL 35 if present, will bind to the antibody pre-coated wells. In order to quantitatively determine the amount of IL 35 present in the sample, a standardized preparation of horseradish peroxidase (HRP)-conjugated polyclonal antibody, specific for IL 35 are added to each well to “sandwich” the IL 35 immobilized on the plate. The microtiter plate undergoes incubation, and then the wells are thoroughly washed to remove all unbound components. Next, substrate solutions are added to each well. The enzyme (HRP) and substrate are allowed to react over a short incubation period. Only those wells that contain IL 35 and enzyme-conjugated antibody will exhibit a change in color. The enzyme-substrate reaction is terminated by addition of a sulphuric acid solution and the color change is measured spectrophotometrically at a wavelength of 450 nm. A standard curve is plotted relating the intensity of the color (O.D.) to the concentration of standards. The IL 35 concentration in each sample is interpolated from this standard curve. |
Coefficient of Variance | Intra Variation% <10% | |
Inter Variation% <12% | ||
Recovery | 95-102% | |
Linearity | Diluent Ratio | Range % |
1:2 | 93-105 | |
1:4 | 88-106 | |
1:8 | 86-108 | |
Specificity/Cross-reactivity | No significant cross-reactivity or interference between IL 35 and analogues was observed. | |



E01I0389 has been referenced in the below publications:
Elevated serum IL-35 and increased expression of IL-35-p35 or -EBI3 in CD4+CD25+ T cells in patients with active tuberculosis.
Assessing the role of IL-35 in colorectal cancer progression and prognosis.
Epstein-Barr Virus-Induced Gene 3 (EBI3) Blocking Leads to Induce Antitumor Cytotoxic T Lymphocyte Response and Suppress Tumor Growth in Colorectal Cancer by Bidirectional Reciprocal-Regulation STAT3 Signaling Pathway.
Study of glucagon-like peptide-2, interleukin 35, and CD8+T cell dysregulation in ulcerative colitis.
Serum IL-35 Levels Are Associated With Activity and Progression of Sarcoidosis.
Reduced IL-35 levels are associated with increased platelet aggregation and activation in patients with acute graft-versus-host disease after allogeneic hematopoietic stem cell transplantation.
Related Bluegene Biotech Products